Bioassay Analysis
|
UNISTAT’s optional Analysis of Bioassays module is a comprehensive implementation of United States Pharmacopoeia chapters <111>, <1033>, <1034> and <81> (2010) and European Pharmacopoeia (1997-2017) Statistical Analysis of Results of Biological Assays and Tests. It is also validated against Finney, D. J. (1978), Statistical Method in Biological Assay and is ready for use in a regulated environment. UNISTAT’s bioassay methods are widely used for biological research and drug development.
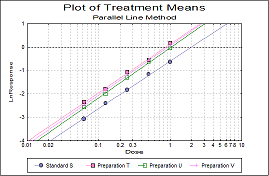
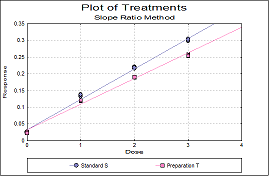
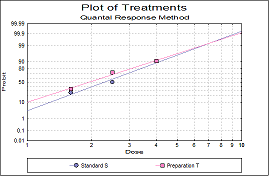
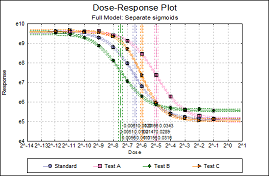
Statistical analysis of data from biological dilution assays or potency assays: Potency calculations can be performed employing parallel line, slope ratio, quantal response or four-parameter logistic model, complete with fiducial confidence intervals, validity tests, potency, ED50, Spearman-Karber method and graphical representations.
The optional Analysis of Bioassays module requires UNISTAT Standard Edition to run. Please contact Unistat Ltd for a quotation.
Major new features in Version 10 include:
|